Ingredients Of A Calcareous Habitat - A Geologist’s Perspective
In this note, we look at the process of removing, or degassing, carbon dioxide gas from groundwater as a means to create a limy deposit, or substrate. We use that insight to explain the formation of some common, calcareous habitats. The formation of calcareous habitats is interesting, and important, because those limy substrates are home to many specialized, calcicolous plants and other specialized life forms.
Calcareous means mostly, or partly, composed of the limy calcium carbonate, which is rich in the element calcium. A calcareous substrate is soil, rock, or chemical precipitate that is rich in calcium carbonate. The calcium carbonate is generally in the form of the mineral calcite. Other minerals, like aragonite, or sometimes calcium sulphate, known as gypsum, can also form a calcareous substrate. Water associated with the formation of some calcareous substrates is generally alkaline, often with a high pH (>8). The water is meteoric in origin derived from rain, melted snow, a creek, or a shallow lake.
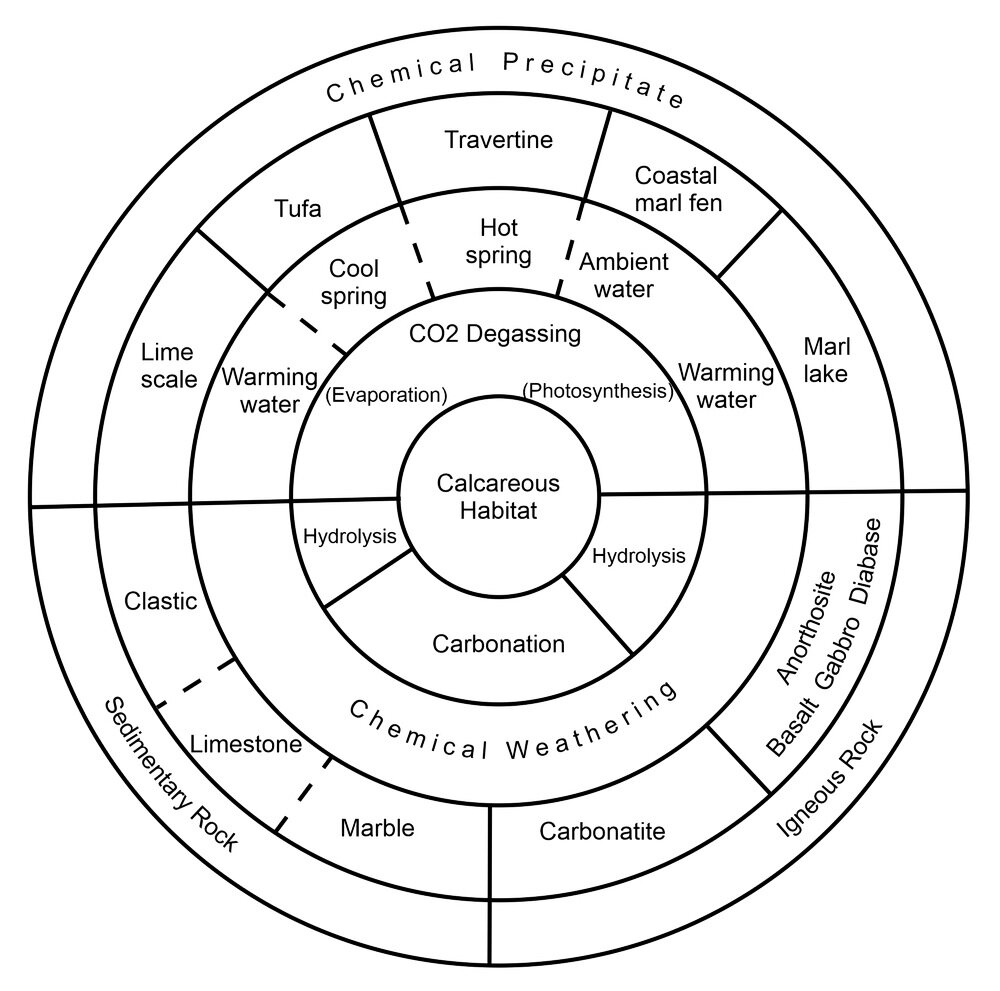
In nature, calcareous substrates form by different natural processes and help create different types of habitats (Photo 1). A sedimentary, limy rock, like limestone or dolomite, exposed at the land surface, is one form of calcareous substrate. A chemical precipitate, like tufa, exposed on the surface is another form of calcareous substrate. Despite the different processes that create a calcareous substrate, they all have one thing in common: lots of calcium in the form of minerals like calcite, aragonite, and sometimes gypsum.
Photo 1: An illustration of the processes by which different geological materials are transformed into a calcareous habitat. Example 1: The igneous rock named basalt can be chemically weathered by hydrolysis to create a calcareous habitat. Example 2: A chemical precipitate can be created in a marl lake by warming of lake water, or photosynthesis, both of which decrease the concentration of carbon dioxide in the lake water, which precipitates calcite and creates a calcareous habitat. Image by Libby Ginn, 2020.
Calcareous habitats include: a) limestone or dolomite alvar; b) calcareous coastal fen; c) marl lake and its shore; d) chemically precipitated flowstone, tufa or travertine; e) chemically precipitated calcite-rich alkali flat; and f) chemically precipitated limescale.
Chemical Notations: The Slightly Boring Bit
Because I cannot display chemical expressions in standard chemical notation within the body of the text, I provide a summary here:
Augite pyroxene: ((Ca,Na)(Mg,Fe,Al,Ti)(Si,Al)2O6)
Anorthite plagioclase feldspar: (CaAl2Si2O8)
Bicarbonate: HCO3-
Calcite: CaCO3
Calcium: Ca
Calcium Sulphate: CaSO4·2H2O
Carbon dioxide: CO2
Carbonate: CO32-
Carbonic acid: H2CO3
Gypsum: CaSO4·2H2O
Water: H2O
Calcareous Ingredients: The More Interesting Bit
Four chemical ingredients are required to make a calcareous substrate:
water;
carbon dioxide gas dissolved in the water;
calcium dissolved in the water; and
a calcium source.
One or more natural processes precipitate the dissolved calcium as calcium carbonate, in the form of the mineral calcite, or as calcium sulphate, in the form of the mineral gypsum. In this note, I focus on some of the geological and geochemical processes that create different calcareous habitats composed of calcium carbonate.
Water source:
Most near-surface water is meteoric in origin, derived from rain, melting snow, lake and river water, or groundwater. Arguably, lake, river and groundwater were ultimately derived from meteoric water. Therefore, for simplicity, I refer to the water associated with the formation of terrestrial calcareous substrates as meteoric water.
If meteoric groundwater circulates at depths less than 0.5 km below the Earth’s surface, the water temperature will remain cool, between 4C and perhaps 25C. If groundwater circulates at depths greater than 1 km below the Earth’s surface, the water will heat up and may reach temperatures greater than 25C. The heat required to warm the groundwater can come from a cooling body of hot igneous rock, like granite, or from the natural heat within the Earth, called the geothermal gradient. For example, the subsurface rock temperature increases by about 25C with each kilometre depth.
Carbon dioxide gas source:
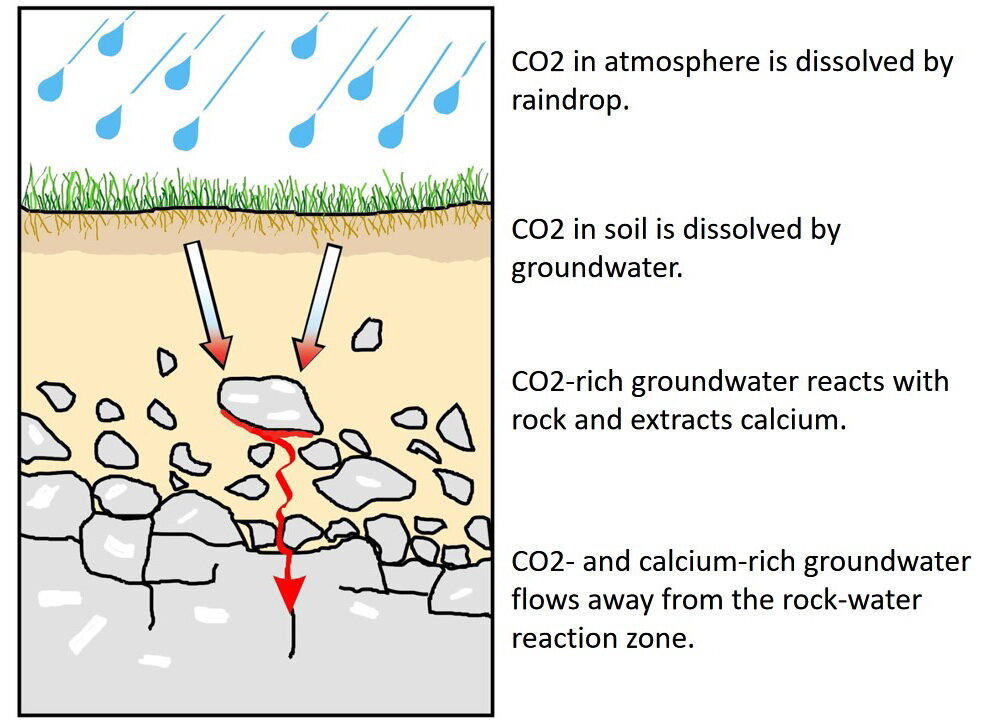
At, and near, the surface of the Earth, carbon dioxide gas is available from two common sources (Photo 2): a) atmosphere; and b) soil. When a raindrop falls through the air, it dissolves some atmospheric carbon dioxide gas. Once on the ground, rainwater, or melted snow, moves into the soil where the water dissolves additional carbon dioxide, present as soil gas, which is produced from decaying organic matter, plant photosynthesis, and respiration by soil bacteria.
Photo 2: Carbon dioxide gas is available from: a) the atmosphere, where it is dissolved in raindrops; and b) soil gas, where it is dissolved in groundwater. As the carbon dioxide charged water percolates down into the rock, that water begins to react with, and to weather, the rocks. That weathering releases the element calcium from the rock-forming minerals. The calcium is carried away by the groundwater to a location where other chemical reactions cause the calcium to precipitate as calcite. That calcite is the foundation for a calcareous substrate and habitat. Cartoon by L. Ginn, 2021.
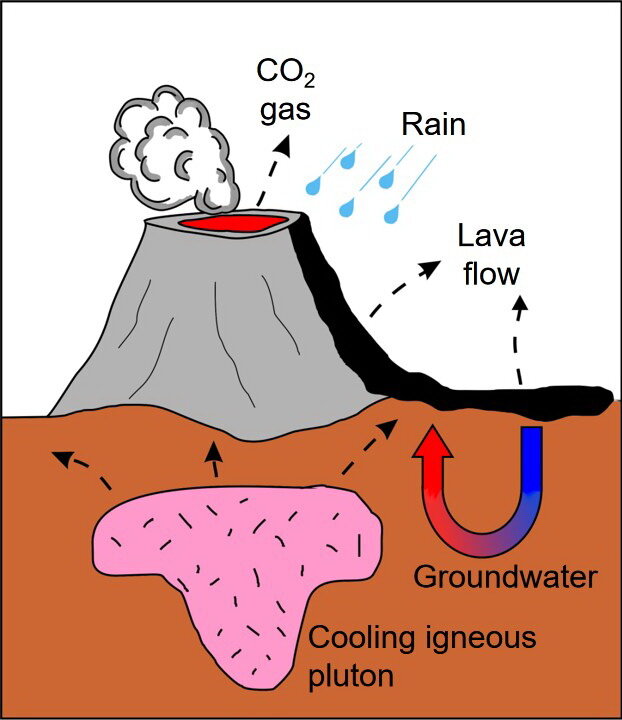
A less common source of carbon dioxide gas is igneous, or juvenile, gas. Igneous carbon dioxide gas comes from degassing of melted rock, also known as magma, or from an igneous rock as it cools and turns from a liquid magma into a solid rock, like a cooling granite or freshly erupted basalt lava (Photo 3). Areas near active volcanos are exposed to abundant igneous carbon dioxide gas.
Photo 3: Cartoon illustrating sources of juvenile or magmatic or igneous carbon dioxide gas. The dashed arrows show the release of carbon dioxide gas from: the top of a volcano; b) a cooling volcanic deposit, such as a lava flow or pyroclastic deposit on he Earth’s surface; and c) a cooling pluton that has solidified below the land surface. Meteoric water falls on the land, sinks into the subsurface where the water takes carbon dioxide gas into solution. Cartoon by L. Ginn, 2021.
Calcium source:
An important calcium source is pre-existing rock that contains the element calcium in its rock-forming minerals. The sedimentary rock limestone and the igneous rock basalt are both potential calcium sources.
Limestone sedimentary rock consists almost entirely of the mineral calcite, which contains a lot of calcium. When exposed on the land surface. water can dissolve limestone to create pits, crevices or caves. A large area of limestone that has reacted with water is called a karst landscape. The water carrying the dissolved calcium flows away from the reaction zone. Where conditions change, the dissolved calcium is chemically precipitated as calcium carbonate to create a calcareous substrate, like tufa, which in turn forms a plant habitat. Even limestone rock exposed on the land surface often forms a calcareous alvar habitat.
Igneous rocks like basalt, gabbro or diabase are made of primary minerals that contain calcium in their original mineral structures. The minerals anorthite plagioclase feldspar (Ca + Al + Si + O) and augite pyroxene (Ca + Na + Mg + Fe + Al + Ti + Si + O) are two such primary rock-forming minerals. Those minerals can be a calcium source under special conditions (Photo 2), discussed in the next section.
Processes That Extract Calcium From A Source Rock
How is calcium extracted from the original rock-forming minerals in a source rock? Chemistry is the answer.
One common chemical reaction that extracts calcium from pre-existing rock is called chemical weathering by hydrolysis (reaction 2, below). When carbon dioxide gas is dissolved in water, chemical reactions produce: a) weak carbonic acid (reaction 1); b) bicarbonate ions; and c) carbonate ions:
[1] : Carbon dioxide gas dissolves in water:
CO2 gas + H2O water = H2CO3 water
carbon dioxide (gas) + water = carbonic acid
Chemists call this water mixture an aqueous solution. The amounts of different ingredients in the aqueous solution depend on factors like water temperature, pH, which is a measure of how acid the water is, and pressure, which is one influence on the amount of carbon dioxide gas dissolved in the water. When this carbon dioxide-bearing aqueous solution interacts with a rock, the weak carbonic acid attacks the primary rock-forming minerals. Calcium in the primary rock-forming minerals is liberated from the mineral structure during the chemical attack (Photo 2; reaction 2):
[2]: Hydrolysis weathering of anorthite plagioclase:
CaAl2Si2O8 + 5H2O + 2H2CO3 = Ca2+ + Al2Si2O5(OH)4 + 2HCO3- + 2SiO2 + 4H2
Plagioclase + 5water +2carbonic acid = calcium ions + clay mineral + 2bicarbonate ions + 2quartz + 4hydrogen gas
For limestone, where the primary mineral is calcite, the chemical reaction is called carbonation. Limestone carbonation begins when water dissolves carbon dioxide gas from the atmosphere and soil to form carbonic acid (reaction 1):
[1]: H2O + CO2 = H2CO3
The carbonic acid in the aqueous solution reacts with, and dissolves, the calcite mineral in the limestone rock, turning it into calcium ions and bicarbonate ions (reaction 3):
[3]: CaCO3 + H2CO3 = Ca2+ + 2HCO3-
Calcite + carbonic acid = calcium ions + 2bicarbonate ions
This is the process that creates karst pits, crevices and caves in limestone rock.
In both chemical weathering by hydrolysis and carbonation, the aqueous solution responsible for chemically attacking the rock also carries away the dissolved calcium ions. The calcium ions remain in the aqueous solution until another chemical reaction causes calcium carbonate (calcite) to precipitate (see below).
Processes That Precipitate Calcium Carbonate
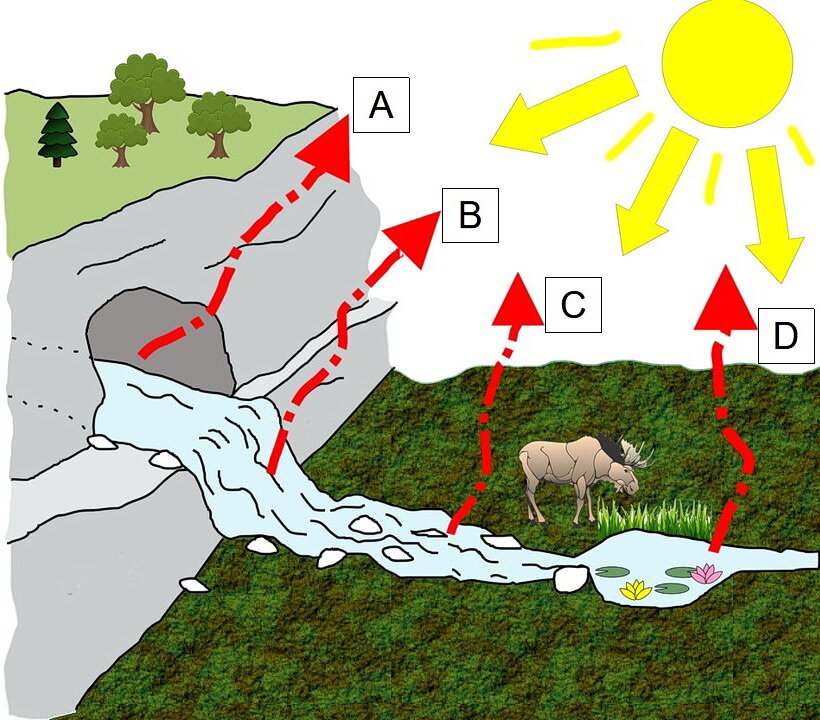
The loss of carbon dioxide gas from an aqueous solution by degassing is a very common way to precipitate calcite. An aqueous solution loses dissolved carbon dioxide gas by several processes, including (Photo 4): a) agitation of the aqueous solution as it flows over an irregular land surface; b) evaporation of the aqueous solution; c) warming of the aqueous solution; d) adjustment of the carbon dioxide gas pressure in the aqueous solution with the carbon dioxide gas pressure in the surrounding environment; and e) plant and bacteria photosynthesis.
Photo 4: An water solution can degas, or lose, dissolved carbon dioxide gas by: A) adjustment of the carbon dioxide gas pressure in the water with the carbon dioxide gas pressure in the surrounding environment, such as inside a cave; B and C) agitation of the aqueous solution as it flows over an irregular land surface; D) evaporation of the aqueous solution and warming of the aqueous solution; and plant and bacteria photosynthesis. Cartoon by L. Ginn 2021
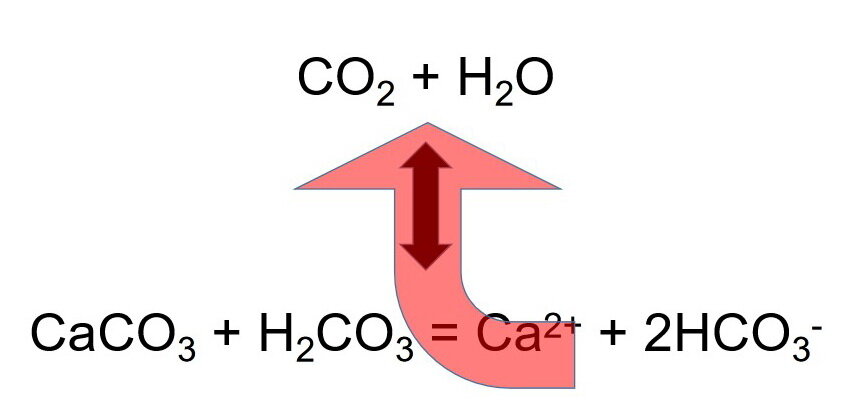
When combined, chemical reactions 1 and 3 illustrate the dissolution or precipitation of calcite depending if carbon dioxide gas is dissolved in, or degassed from, the aqueous solution (Photo 5):
[1]: CO2 gas + H2O water = H2CO3
[3]: CaCO3 + H2CO3 = Ca2+ + 2HCO3-
when combined, give chemical reaction [4]:
Photo 5: This chemical reaction illustrates what happens to an aqueous solution that is saturated with carbon dioxide gas and is in equilibrium with the mineral calcite. If carbon dioxide gas is removed from the solution (top line), the chemistry attempts to compensate by following the path of the red arrow by combining calcium and bicarbonate ions to form the mineral calcite, carbon dioxide gas and water.
Chemical reaction [4] tells us when carbon dioxide gas is lost, or removed, or degassed, from the aqueous solution, the chemical reaction moves in the direction of the red arrow. That causes the mineral calcite to precipitate. The precipitated calcite accumulates as a calcareous bed, layer, or mound of calcite on the land surface.
Generally, that calcareous substrate forms close to the zone where the aqueous solution, such as a groundwater spring, discharges onto the land surface. If a water pool or lake is formed at the discharge site, sometimes you see bubbles of carbon dioxide gas rising to the water surface (Photo 6 and 7). Those bubbles confirm that carbon dioxide gas is degassing from the water into the atmosphere. Calcite can also precipitate as limescale when the aqueous solution warms as it flows over a surface heated by the Sun, or evaporates as it flows over the land surface.
Photo 6: Carbon dioxide bubbles breaking the surface of a small pool, fed by warm, carbon dioxide-charged, lime-rich groundwater spring is evidence of carbon dioxide degassing. This degassing created a tufa deposit because calcite is precipitated. Photo composed by Andy Fyon at the Warm Springs pool, south of Atlin, northern British Columbia, July 19/08.
Photo 7: Carbon dioxide bubbles breaking the surface of the Alpha Pool, Liard River Hot Springs, which is fed by hot, carbon dioxide-charged springs. The bubbling is evidence of carbon dioxide degassing. Calcite precipitates as a result. That calcite has created tufa deposits and a large calcareous fen in this area. Photo composed by Andy Fyon at Liard River Hot Springs Provincial Park, July 21, 2008.
A calcareous habitat can be created on, and near, any rock that contains calcium when that rock is chemically weathered by carbon dioxide-bearing meteoric water or when a limestone or dolomite rock undergoes carbonation.
Relevance To The Formation Of Different Calcareous Habitats
We can apply the process of carbon dioxide degassing to explain one way to create some common calcareous habitats by precipitation of the mineral calcite.
Marl lake:
Photosynthesis by algae, like Chara, and other aquatic plants, can cause precipitation of calcite in lake, pond, or river water. Photosynthesis removes carbon dioxide gas from the water. Loss of carbon dioxide gas causes calcite to precipitate. That calcite accumulates on some of the Chara extremities (Photo 8), on the lake bottom (Photo 9), and around the lake shore (Photo 10). Warming of cold, carbon dioxide-saturated lake water over the summer is another way to precipitate calcite. Warm water holds less dissolved carbon dioxide gas in solution compared to cold lake water. Warming lake water, such as at Little Limestone Lake, Manitoba, causes carbon dioxide gas to degas from the water. The loss of dissolved carbon dioxide gas, as the water warms, causes calcite to precipitate. The precipitated calcite creates calcareous deposits in, and around, the lakeshore to create a marl lake and a calcareous riparian habitat adjacent to the marl lake.
Photo 8: Common Stonewort, inferred to be Chara vulgaris, grows in a calcareous fen at Liard River Hot Springs Provincial Park. The white colour on the outside of some appendages is reported to be calcareous deposits, based on the information signs at the park. Image by Andy Fyon, Liard River Hot Springs Provincial Park, British Columbia, June 5/19.
Photo 9: Stoneworts (Genus Chara) growing in a shallow marl lake, named Turtle Pond, at MacGregor Point Provincial Park, Ontario. The light brown colour is reported on the information signs to be calcareous material. Photosynthesis by algae (Chara) removes carbon dioxide from the carbon dioxide-charged, limy water. That carbon dioxide degassing causes the calcareous material to precipitate. Image by Andy Fyon, June 2/18.
Photo 10: A number of marl lakes, like this one, are marked by light coloured calcareous material and algae along the lakeshore and on the lake bottom. I speculate that algae photosynthesis and warming of the lake water are responsible for degassing of carbon dioxide from the lake water and precipitation of the calcareous material. Image by Andy Fyon, north of Fort Providence, along the side of Highway 3, Northwest Territories, July 1/13.
Calcareous coastal fen:
In the Great Lake basins of Lake Ontario, Lake Erie, Lake Huron and Lake Michigan, coastal, calcareous fen wetlands occur adjacent to areas underlain by limestone and dolomite rock. The coastal marsh at Oliphant is one such example (Photo 11).
Photo 11: Oliphant coastal marsh is considered to be a calcareous fen. Carbon dioxide- and calcium-charged groundwater likely contributed to the water in the wetland. Degassing of carbon dioxide from the water may have been caused by one, or all, of warming and evaporation of the shallow marsh water, photosynthesis by plants and algae, and transfer of carbon dioxide gas from the groundwater into the atmosphere because the groundwater contained more carbon dioxide gas than the atmosphere. Degassing of carbon dioxide gas from the water caused the precipitation of calcareous material, which gives the floor of the wetland a light brown colour. Image by Andy Fyon, at the Oliphant coastal fen wetland, Bruce Peninsula, Ontario, June 1/18
Below the land surface, through the process of carbonation [see above], groundwater reacted with the limestone and dolomite rock to become saturated with dissolved carbon dioxide gas and calcium. Three processes, acting together or alone, lead to degassing of carbon dioxide gas from the groundwater as it emerged onto the land surface (Photo 12):
photosynthesis by algae, bacteria, and aquatic plants, which removes carbon dioxide gas from the calcium-rich fen water;
warming, by the Sun, of the shallow, calcium-rich fen water caused degassing of carbon dioxide gas from the carbon dioxide- and calcium-rich recharge groundwater; and
transfer of carbon dioxide gas from the groundwater into the atmosphere because the groundwater contained more carbon dioxide gas than the atmosphere.
Photo 12: A cartoon that illustrates one way the create a calcareous, coastal fen or wetland. Carbon dioxide- and calcium-rich groundwater (light blue arrows) flows through limestone and dolomite rocks (grey colour) and discharges onto to the shore area to help create a coastal marsh (light blue colour). Marsh plants and algae (black marks) occur in the coastal marsh. A sandy beach area is coloured yellow. Several processes can cause calcite to precipitate, which is the chemical foundation to a calcareous coastal fen: [1] photosynthesis by algae, bacteria, and aquatic plants, which removes carbon dioxide gas from the calcium-rich fen water; [2] warming of the shallow surface water by the Sun caused degassing of carbon dioxide gas from the groundwater; and [3] carbon dioxide degassing from the groundwater when it discharges on the land surface because the groundwater contains more carbon dioxide gas than the atmosphere.. Cartoon by L. Ginn, 2021.
That loss of carbon dioxide gas caused calcite to precipitate in the riparian and the lakeshore wetland areas adjacent to the lakes, where the fens are recharged by the emerging groundwater. The precipitated calcite is the calcareous foundation of the coastal fen habitat.
Limescale:
Limescale (Photo 13) can form indirectly from chemical weathering of a calcium-bearing rock by hydrolysis. For example, chemical weathering of the igneous rocks basalt, gabbro or diabase breaks down the original rock-forming minerals like anorthite plagioclase and augite pyroxene. Those original minerals contain calcium in the structure. The weathering releases calcium from the original minerals. The aqueous solution, responsible for weathering, dissolves the liberated calcium and transports it away from the weathering zone. Limescale forms when the aqueous solution flows, or seeps, over the land surface and loses carbon dioxide gas by degassing, evaporation, and/or solution warming.
Photo 13: The red arrows point to white-coloured limescale calcite that precipitated on the surface of a rock outcrop. Image by Andy Fyon, Gargantua trail, Lake Superior Provincial Park, Ontario, Aug. 15/18.
Flowstone, tufa and travertine:
Tufa (Photo 14), flowstone, and travertine are calcareous substrates that form when calcite precipitates from groundwater. The calcite accumulates as a layer, bed or mound where the groundwater spring discharges onto, and flows along, the land surface. Generally, the pressure of carbon dioxide gas dissolved in groundwater is higher than the pressure of carbon dioxide gas contained in the atmosphere. When that groundwater is exposed to the air, in a cave or on the land surface, carbon dioxide gas degasses from the mineral spring. That loss of dissolved carbon dioxide gas causes calcite to precipitate. The calcite deposits occur as layers and speleothems in caves (e.g., flowstone) or mounds on surface (e.g., tufa and travertine), which creates calcareous habitats.
Photo 14: A tufa deposit forms where a carbon dioxide-charged, lime-rich groundwater spring discharges onto the land surface. Carbon dioxide gas degassing from the groundwater, is one factor causing precipitation of calcite - the main component of the tufa. Image by Andy Fyon, composed at Warm Spring Bay, Atlin, British Columbia, July 17/19.
Calcite alkali flat:
Calcite-rich, alkali flats (Photo 15) can form when carbon dioxide- and calcium-rich groundwater seeps towards the land surface and degasses carbon dioxide.
Photo 15: A large area of calcite alkali flat near Carcross, Yukon. The white coloured material is the precipitated calcite. Image by Andy Fyon, July 22/19.
An alkali flat is a level area that is covered by alkali minerals, such as sodium sulphate or carbonate mineral like calcite. Some alkali flats are created where a lake evaporates more quickly than it is recharged by water. In that case, the alkali flat is called a playa and the flat alkali surface used to be a lake bottom.
An alkali flat can also form where groundwater, saturated with carbon dioxide gas and calcium, discharges onto the land surface, in a low lying area or basin. As the groundwater seeps toward the land surface, it degasses carbon dioxide gas because: a) the carbon dioxide gas pressure in the groundwater exceeds the carbon dioxide gas pressure in the atmosphere; b) the groundwater saturates the surface materials, which is warmed by the Sun; or c) the groundwater evaporates. In the Carcross example, the presence of polygonal desiccation features in the porous, wet calcite deposit suggests the calcite did not form by the evaporation of an alkali lake and therefore, the calcite deposit does not represent a playa, or dry lake bottom. Rather, I speculate that calcite precipitated in this area underlain by permafrost when calcium-rich, cold groundwater, saturated with carbon dioxide gas, seeped to the land surface, where carbon dioxide gas degassed. The loss of the carbon dioxide gas causes calcite to precipitate, which created the surface alkali flat. The alkali flat may also grow upward if the calcite precipitates below the surface. The precipitated calcite substrate creates the calcareous habitat.
Summary
Meteoric water, carbon dioxide gas dissolved in the water, and calcium, derived from local rock by reaction with the water, are important chemical ingredients required to make a calcareous substrate. Degassing of a carbon dioxide- and calcium-rich groundwater system precipitates calcite from the water. The limy, calcium-rich deposit formed by the precipitated calcite is the foundation for a calcareous habitat, like a marl lake, tufa, calcite-rich alkali flat, coastal lime-rich wetland fen, and limescale. Those limy habitats are home to specialized calcicolous plants, birds, insects and other life forms.
Have A Question About This Note?
Jan 31, 2021