Geochemistry: Limescale
Calcareous habitats are unusual and uncommon. They are home to some unusual flora, birds, and insects. Understanding the geological and geochemical processes that create a calcareous habitat helps inform us about factors that influence the location and physical features of a calcareous habitat and the types and distributions of flora that live on a calcareous habitat.
Limescale On A Rock Surface
Limescale is likely something you have encountered, but perhaps did not recognize. Limescale is a chalky white- or tan-coloured deposit of the mineral calcite (CaCO3). You can usually scratch flakes of limescale with your fingernail and can certainly scratch it with a knife. Common places where limescale builds up in our homes includes the inside of kettles, hot water boilers, and pipes. In nature, you may have seen limescale as a white chalky coating on the surface of an exposed rock (Photo 1).
Photo 1: The red arrows point to white-coloured limescale on the surface of a rock called diabase dike. The limescale formed from calcium-rich seepage that drained out from underground, to the surface, through a network of cracks and fractures in the rock. Image by Andy Fyon, Gargantua trail, Lake Superior Provincial Park, Aug. 15, 2018.
The calcite material may also fill cracks and fractures in a rock. In this form, the calcite may look like a white vein (Photo 2).
Photo 2: Red arrows point to calcite that fills cracks and fractures in a diabase rock. The calcite formed when calcium-rich groundwater flowed underground through a network of fractures in the rock on its journey to the Earth’s surface. Image by Andy Fyon, Gargantua trail, Lake Superior Provincial Park, Aug. 15, 2018.
Sometimes limescale acts as a type of “cement” binding cobbles together within a gravel deposit or an ancient conglomerate (Photo 3).
Photo 3: The red arrows point to calcite limescale that binds cobbles together in this ancient conglomerate deposits. Image by Andy Fyon, of a Keweenawan conglomerate, at Deadmans Cove, Aug. 21, 2018.
Limescale Formation: Temperature Increase
How does calcite limescale form? Calcite limescale can precipitate on the surface of a warm rock when cold, calcium- and CO2-bearing groundwater flows out over the sun-baked rock surface (Photo 4).
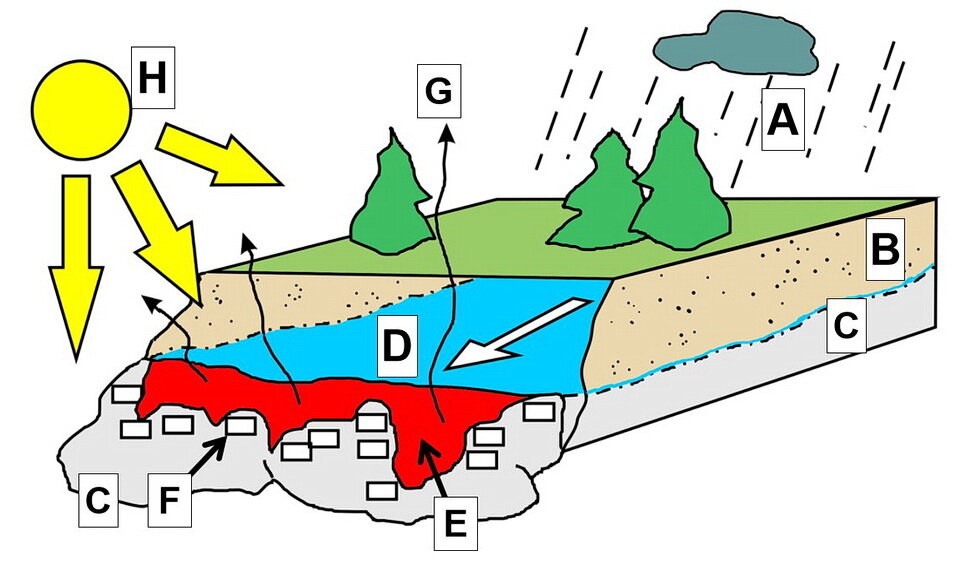
Photo 4: Conditions which lead to the formation of calcite limescale on the warm surface of a Sun-baked rock. Letters mean: A: rain or snow source of groundwater; B: soil; C: rock; D: cold groundwater containing dissolved calcium and carbon dioxide (CO2); E: groundwater warmed by the Sun as the water flows over the Sun-bakes rock surface; F: calcite limescale or calcite infilling cracks and fractures in the rock; G: Evapouration of the warm calcium- and CO2-bearing groundwater; and H: Sun. Image by E. Ginn.
On the rock surface, the temperature of groundwater increases. Warm water contains much less dissolved carbon dioxide (CO2) gas compared to cold water. Therefore, as the groundwater warms, it releases some of the dissolved CO2 gas.
I provide a more detailed discussion of the chemical reactions at play between water (H2O), carbon dioxide (CO2) gas, carbonic acid (H2CO3) and calcite in another note where I discuss the simple conditions of calcite precipitation. Simply, the loss of CO2 gas from the groundwater causes chemical reaction [3] shifts to the left to try to compensate for loss of CO2.
H2O + CO2 = H2CO3 [3]
That consumes, or reduces, the amount of carbonic acid (H2CO3) dissolved in the groundwater solution. The loss of carbonic acid (H2CO3) causes reaction [2] to compensate by shifting to the left to produce more carbonic acid (H2CO3), which causes calcite (CaCO3) to precipitate:
CaCO3 + H2CO3 = Ca+2 + 2HCO-3 [2]
That is one explanation why calcite limescale forms on the rock surface when cold calcium- and CO2-bearing groundwater flows over the warm surface of a Sun-baked rock (Photo 4).
Limescale Formation: Evapouration
Another process that causes calcite to precipitate is the evapouration of the groundwater as it flows over the warm rock surface (Photo 4). Evapouration leads to the loss of dissolved carbon dioxide (CO2) from the groundwater, which leads to calcite precipitation.
Limescale Formation: Pressure Decrease
When groundwater flows through cracks and fractures in rock deep in the Earth, the water is able to contain more dissolved carbon dioxide (CO2) compared to water on the Earth’s surface because the pressure acting on the solution deep below the Earth’s surface is higher compared to the pressure on the Earth’s surface.
As the groundwater flows from deep below the surface, to the surface, the pressure acting on the groundwater decreases. That pressure decrease causes the groundwater to release some dissolved carbon dioxide (CO2). This causes reaction [3], above, to shift to the left to try to compensate for the lost CO2. That reverse reaction consumes carbonic acid, which causes reaction [2] to shift to the left, which causes calcite to precipitate.
Implication
The formation of limescale coatings on rock surfaces or in seeps create micro-habitats suitable of sustaining specialized, calciphile plants and other lifeforms on rocks, or in areas, that would not be considered to be calcareous. It is not essential for limescale to actually form. Calcareous habitats can also form where calcium-rich seepage discharges from the ground and ponds in an area.
Summary
Limescale, also known as a form of the mineral calcite (CaCO3), can form on the surface of a Sun-baked rock when:
the carbon dioxide (CO2) gas pressure affecting the groundwater solution decreases, say, when the groundwater flows from deep below the Earth’s surface to the surface;
the water temperature of the groundwater solution increases by warming the water as it flows, or seeps, over the Sun-baked rock surface; and
the evapouration of the calcium- and CO2-bearing groundwater solution when it seeps over the exposed, Sun-baked rock surface.
These are three simple ways to explain the formation of limescale coatings on exposed rock surfaces at, or near, the Earth’s surface. The limescale coating, formed from calcareous drainage, creates a micro-habitat suitable of sustaining specialized calciphile plants and other lime-loving lifeforms on rocks, or in areas, that would not normally be considered to be calcareous.
Do you have a question or a comment?
Andy Fyon: Sept. 6/20.