Chemical Weathering Of Diabase And Calcicolous Plants
Is there is a connection between calcicolous plants and non-calciphile rocks that formed deep in the Earth? Yes!
Along the coastal trail, in Lake Superior Provincial Park, I saw many instances where the calcicolous plants Shrubby Cinquefoil (Dasiphora fruticosa) (Photo 1), Common Butterwort (Pinguicula vulgaris) (Photo 2), Bog Lobelia (Lobelia kalmii) (Photo 3), and Mistassini Primrose (Primula mistassinica) (Photo 4) grew on igneous rock called diabase (Photo 5).
Photo 1: Shrubby Cinquefoil (Dasiphora fruticosa) growing on diabase rock that has been chemically weathered. The chemical weathering liberated calcium from the minerals that make up the diabase rock. That calcium precipitated as calcite in fractures that cut the rock. Image by Andy Fyon, Gargantua trail south, Lake Superior Provincial Park, Ontario, Canada, Aug. 15, 2018.
Photo 2: Common Butterwort (Pinguicula vulgaris) growing on diabase rock that has been chemically weathered. The chemical weathering liberated calcium from the minerals that make up the diabase rock. That calcium precipitated as white calcite in fractures that cut the rock to create a micro-calcareous habitat. Photo composed by Andy Fyon, Gargantua trail south, Lake Superior Provincial Park, Ontario, Canada, Aug. 15/18.
Photo 3: Bog Lobelia (Lobelia kalmii) growing on diabase rock that has been chemically weathered. The chemical weathering liberated calcium from the minerals that make up the diabase rock. That calcium precipitated as white calcite in fractures that cut the rock to create a micro-calcareous habitat. Photo composed by Andy Fyon, Gargantua trail south, Lake Superior Provincial Park, Ontario, Canada, Aug. 15/18.
Photo 4: Mistassini Primrose (Primula mistassinica) growing on diabase rock that has been chemically weathered. The chemical weathering liberated calcium from the minerals that make up the diabase rock. That calcium precipitated as white calcite in fractures that cut the rock to create a micro-calcareous habitat. Photo composed by Andy Fyon, Gargantua trail south, Lake Superior Provincial Park, Ontario, Canada, Aug. 15/18.
Photo 5: Image of diabase dike rock exposed along the Coastal Trail, Lake Superior Provincial Park, Ontario, Canada. The white mineral is anorthite plagioclase feldspar (CaAl2Si2O8). The black mineral is augite pyroxene ((Ca,Na)(Mg,Fe,Al,Ti)(Si,Al)2O6). Those minerals formed deep in the Earth and they are not stable on the Earth’s surface, where they undergo chemical weathering. Photo composed Aug. 15/18.
No doubt, some of you have seen that as well and have been sleepless wondering why those calcicolous plants grow on a rock that geologists do not consider to be calcareous. The connection comes from the chemical weathering of two primary minerals that make up diabase: anorthorite plagioclase (CaAl2Si2O8) and augite pyroxene ((Ca,Na)(Mg,Fe,Al,Ti)(Si,Al)2O6).
Chemical Weathering By Hydrolysis
On the Earth’s surface, rocks undergo chemical weathering by hydrolysis when they react with water, carbon dioxide (CO2) dissolved in the water, and carbonic acid (H2CO3). For a more extensive discussion of chemical weathering by hydrolysis, check this page.
Because plagioclase and pyroxene form deep in the Earth, they are not stable on the Earth’s surface, where they are transformed by chemical weathering to secondary minerals. Both anorthite plagioclase and augite pryoxene contain calcium in their mineral structures. During chemical weathering, that calcium is released from the mineral structures. The liberated calcium is generally carried away from the weathering zone by the water solution.
Precipitation Of Calcite
The liberated, aqueous calcium liberated by the chemical weathering of plagioclase and pyroxene precipitates as the mineral calcite (CaCO3) under the right conditions. One simple way to precipitate calcite is reduce the “amount” of CO2 gas dissolved in the water. There are two common ways to reduce the amount of CO2 gas in the water:
water warms as it flows from the cold, subterranean, fractured rock onto the hot, sun-baked rock surface and CO2 degases from that warm water because CO2 is much less soluble in warm water than in cold water; and/or
evapourate the water solution on, or near, the rock surface and the dissolved CO2 gas passes into the atmosphere when the water solution flows onto the rock surface.
Both processes cause calcite to precipitate. Depending on the state of the rock, some calcite will precipitate in fractures in the rock (Photo 6).
Photo 6: The red arrows point to white-coloured calcite (CaCO3) mineral that precipitated in fractures within the altered diabase rock. Image by Andy Fyon, along the Gargantua trail south, Lake Superior Provincial Park, Ontario, Canada, Aug. 15/18.
Larger fractures will channel the water solution to the surface where a calcareous limescale forms on the rock surface due to evapouration of the watery solution (Photo 7).
Photo 7: The red arrows point to a white-coloured coating of calcite (CaCO3) limescale on the surface of a diabase rock. It appears that calcium-bearing water solution seeped onto the rock surface from larger cracks in the rock. Once on surface, evapouration of the water solution caused the limescale to precipitate. Photo by Andy Fyon, along the Gargantua trail south, Lake Superior Provincial Park, Ontario, Canada, Aug. 15/18.
Calcareous Substrate
Both geological process create a calcareous micro-habitat with a limy substrate on, and near, the non-calcareous diabase rock, which can support calciphile plants (Photo 8). Seepage of the limy water away from the rock will create calcareous micro-habitats nearby.
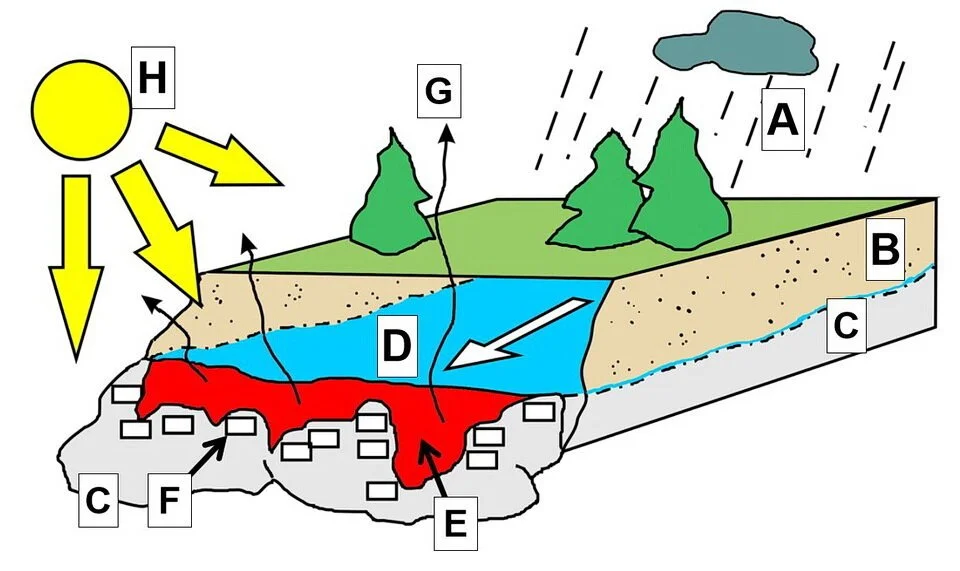
Photo 8: Conditions which lead to the formation of calcite limescale on the warm surface of a Sun-baked rock. Letters mean: A: rain or snow source of groundwater; B: soil; C: rock; D: cold groundwater containing dissolved calcium and carbon dioxide (CO2); E: groundwater warmed by the Sun as the water flows over the Sun-bakes rock surface; F: calcite limescale or calcite infilling cracks and fractures in the rock; G: Evapouration of the warm calcium- and CO2-bearing groundwater; and H: Sun. Image by E. Ginn.
Summary
Chemical weathering of diabase rock will generate limy water, carrying dissolved calcium. The calcium will precipitate as calcite and create a calcareous substrate when the amount of carbon dioxide (CO2) in the water is reduced due to heating of the water on the sun-baked rock surface and / or by evapouration of the CO2-bearing water at, and near, the rock surface. The calcareous micro-habitat with its limy substrate will support calcicolous plants. That is one explanation to account for the presence of calcicolous, or lime-loving, plants on, and close to, a non-calcareous igneous rock.
If you have a comment, contact me via email.
Created: Sept 24/20; Oct 8/20; Sept 13/21; Nov 15/21.